Enhancing Efficacy of Albumin-Bound Paclitaxel for Human Lung and Colorectal Cancers through Autophagy Receptor Sequestosome 1 (SQSTM1)/p62-Mediated Nanodrug Delivery and Cancer therapy (Lin et al., ACS Nano, 2023)
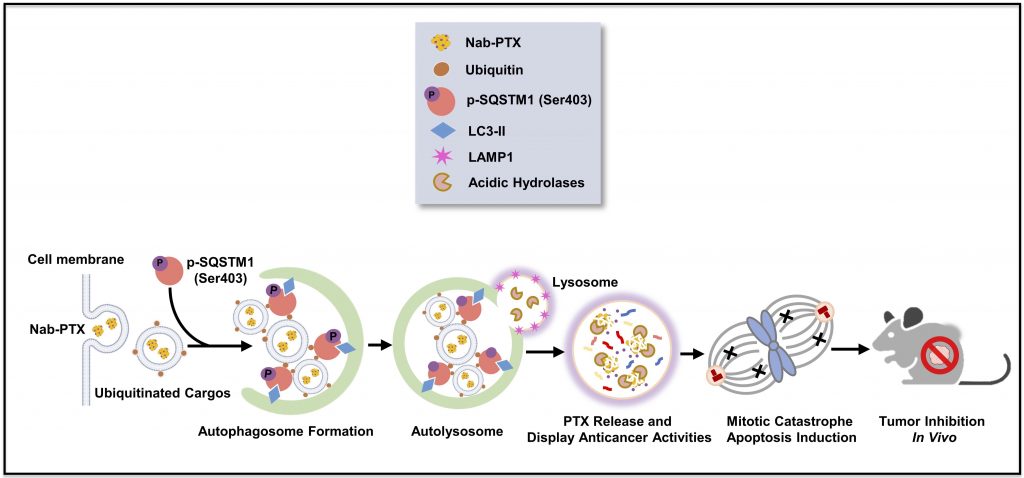
We show that SQSTM1 plays a crucial role in Nab-PTX drug delivery and efficacy in human lung and colorectal cancers. Nab-PTX induces SQSTM1 phosphorylation at Ser403, which facilitates its incorporation into the selective autophagy of nanoparticles, known as nanoparticulophagy. SQSTM1 enhanced Nab-PTX recognition to form autophagosomes, which were delivered to lysosomes for albumin degradation, thereby releasing PTX to induce mitotic catastrophe and apoptosis. SQSTM1 is highly expressed in advanced lung and colorectal tumors and is associated with poor overall survival in clinical patients. Targeting SQSTM1 may provide an important strategy to improve nanodrug efficacy in clinical cancer therapy.
Targeting EGFR and Monitoring Tumorigenesis of Human Lung Cancer Cells In Vitro and In Vivo Using Nanodiamond-Conjugated Specific EGFR Antibody (Lin et al., Pharmaceutics, 2023)
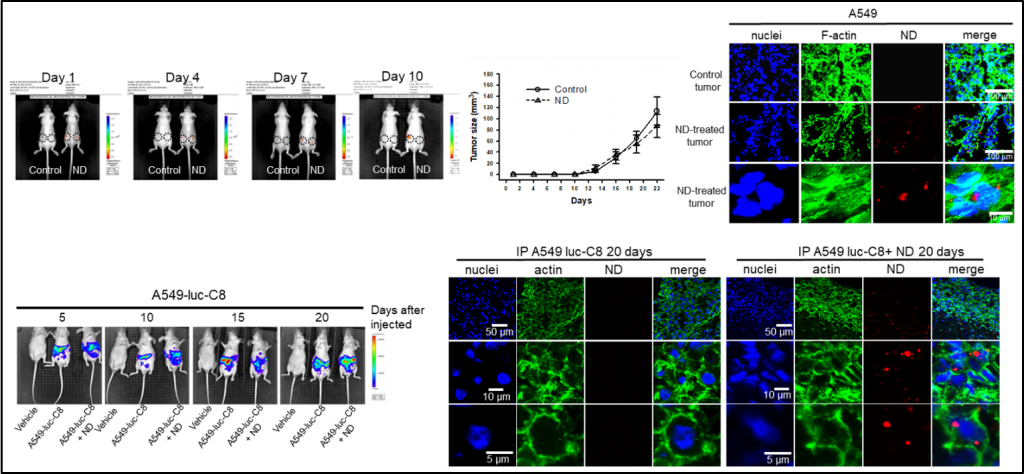
We show a carbon-based nanoprobe, nanodiamond (ND), which can be applied for targeting EGFR and monitoring tumorigenesis of human lung cancer cells in vitro and in vivo. The fluorescence signal of ND particles can be real-time detected in the xenografted human lung tumor formation of nude mice. Moreover, the ND-conjugated specific EGFR antibody cetuximab (Cet) can track the location and distribution of EGFR proteins of lung cancer cells in vitro and in vivo.
Co-inhibition of Aurora A and Haspin kinases enhances survivin blockage and p53 induction for mitotic catastrophe and apoptosis in human colorectal cancer (Lin et al., Biochemical Pharmacology, 2022)
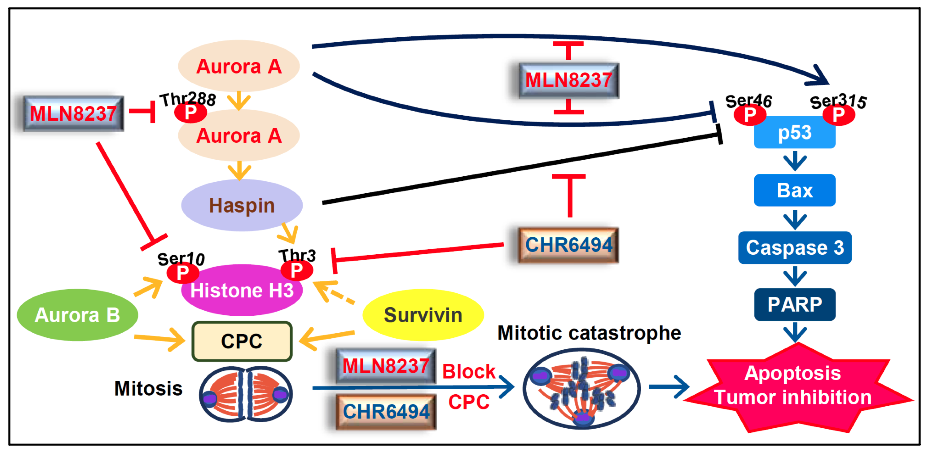
Colorectal cancer (CRC) is a leading cause and mortality worldwide. Aurora A and haspin kinases act pivotal roles in mitotic progression. However, the blockage of Aurora A and Haspin for CRC therapy is still unclear. In this study, co-inhibition of Aurora A and Haspin enhances survivin inhibition, p53 pathway induction, mitotic catastrophe, apoptosis and tumor inhibition that may provide a potential strategy for CRC therapy.
A novel EGFR inhibitor suppresses survivin expression and tumor growth in human gefitinib-resistant EGFR-wild type and -T790M non-small cell lung cancer (Wang et al., Biochemical Pharmacology, 2021)
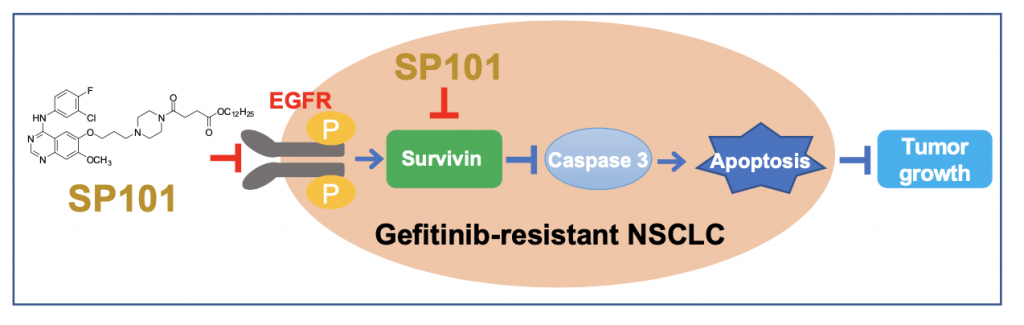
SP101 is a novel EGFR inhibitor that suppresses survivin expression and tumor growth in human gefitinib-resistant EGFR-wild type and -T790M non-small cell lung cancer (NSCLC). SP101 provides a new class EGFR inhibitor by blocking survivin to overcome drug resistance of gefitinib for the NSCLC therapy.
Selective Autophagy Pathway of Nanoparticles and Nanodrugs: Drug Delivery and Pathophysiological Effects (Raj et al., Advanced Therapeutics, 2020)
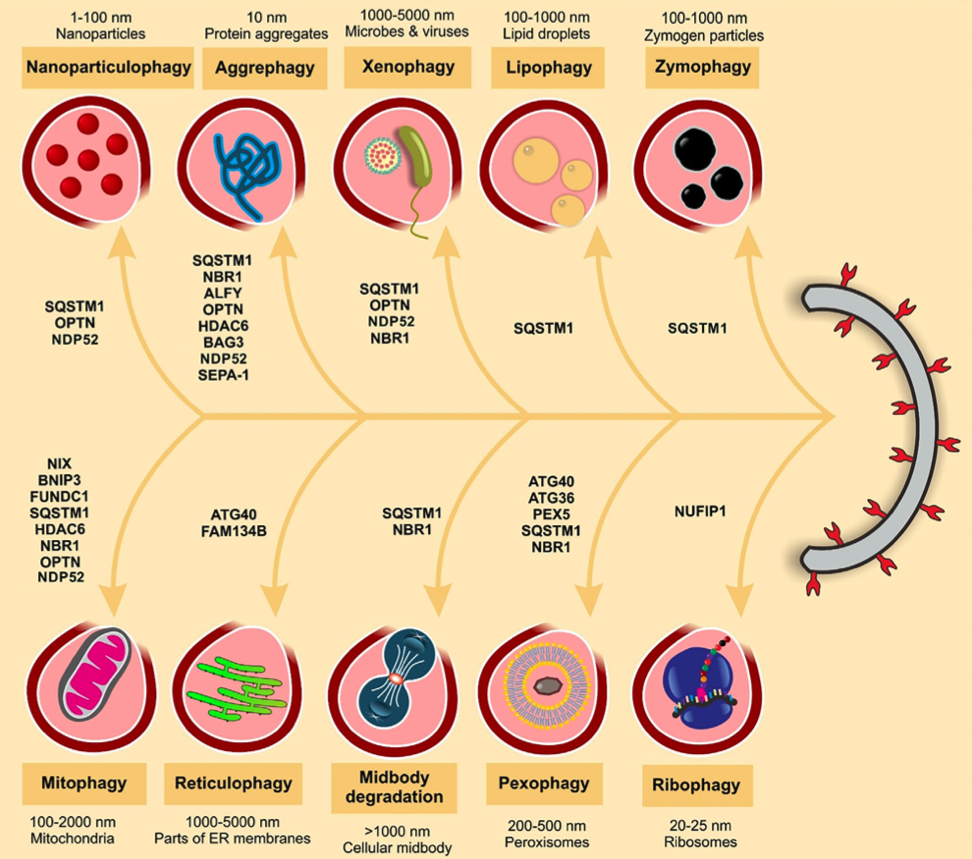
The selective autophagy of nanoparticles and nanodrugs occurs through binding to ubiquitinated proteins, autophagy receptors, and LC3, the formation of nanoparticulosomes, and their delivery to lysosomes. We termed this processing of nanoparticles and nanodrugs through selective autophagy pathways “Nanoparticulophagy”.
Targeting EGFR of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel- and cetuximab-conjugated nanodiamond nanocomposite (Liao et al., Acta Biomaterialia, 2019)
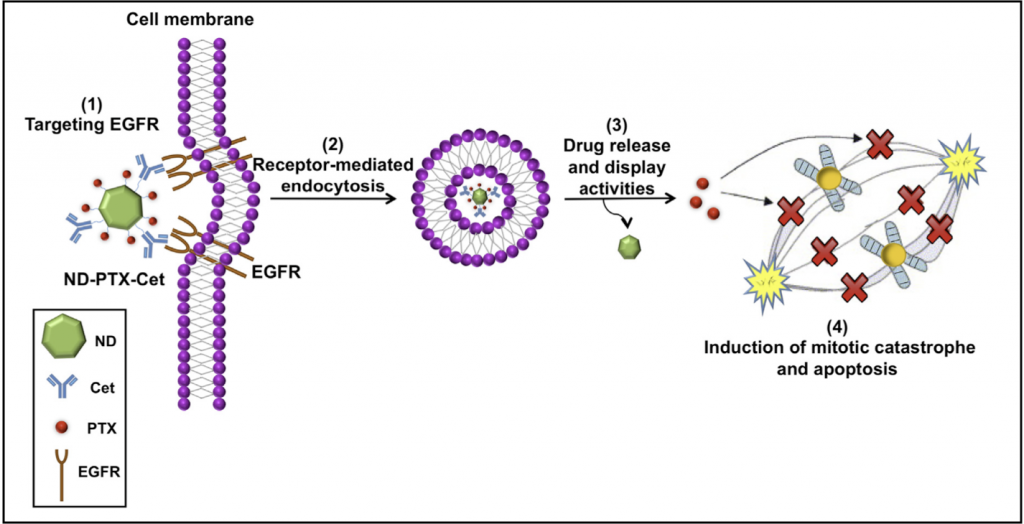
We designed a nanocomposite by using nanodiamond conjugated with paclitaxel and cetuximab (ND-PTX-Cet) for targeting therapy on the EGFR-positive triple-negative breast cancer (TNBC). ND-PTX-Cet nanocomposite enhanced mitotic catastrophe and apoptosis by targeting EGFR of TNBC cells.
Ubiquitin-coated nanodiamonds bind to autophagy receptors for entry into the selective autophagy pathway (Liu et al., Autophagy, 2017)
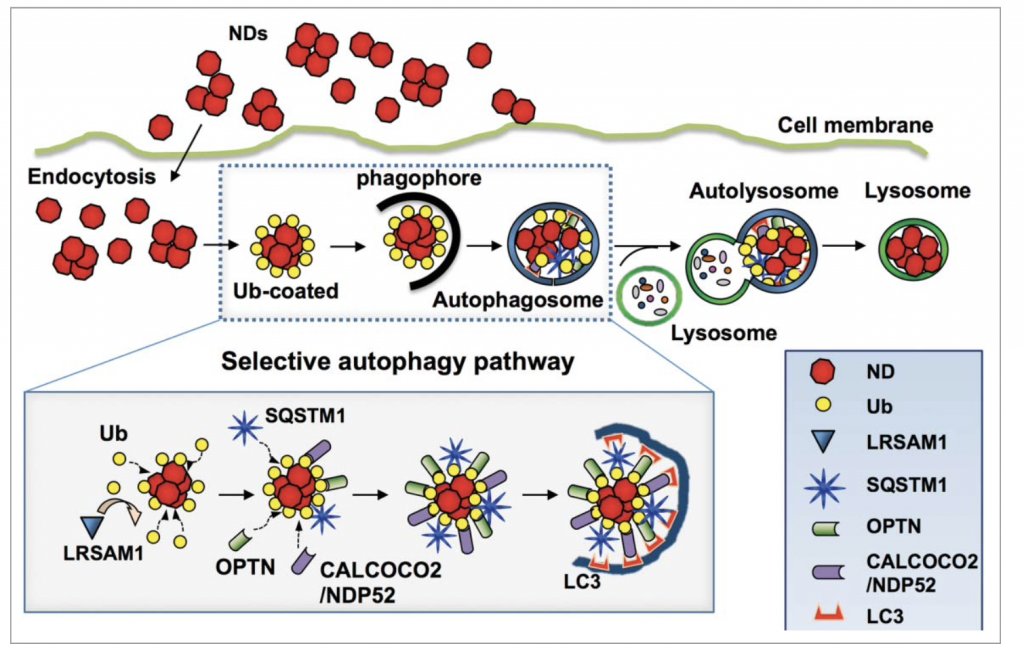
Selective autophagy plays a pivotal role in the processing of foreign pathogens and cellular components to maintain homeostasis in human cells. To date, numerous studies have demonstrated the uptake of nanoparticles by cells, but their intracellular processing through selective autophagy remains unclear. This study demonstrated for the first time that Ub-coated nanoparticles bind to autophagy receptors for entry into the selective autophagy pathway, facilitating their delivery to lysosomes.